What is a cytokine storm?
Cytokine Release Syndrome (CRS), otherwise known as cytokine storm, is a systemic inflammatory response caused by complications due to disease, infection or an adverse effect of biologic therapy. The clinical symptoms of a cytokine storm are massive release of a potent cocktail of pro-inflammatory cytokines into the general circulatory system, leading to severe multi-organ damage, failure or potentially death. This is an extremely unwanted immunotoxicological side effect in drug development.
One of the best documented cases of pharmaceutical-related CRS is the TGN1412 clinical trial undertaken in 2006. This monoclonal antibody therapy targeted against CD28 receptor was designed to treat haematological cancers and autoimmune disease. During preclinical safety testing, study findings indicated no serious adverse events, even while dosing at ~500x the safe clinical trial dose. These findings were not reflected in the clinical trial. All subjects administered TGN1412 experienced infusion-related reaction leading to cytokine storm. As a result, less reliance is placed on animal models to identify and highlight the potential risk of dosing with novel biologic compounds. The TGN1412 trial is not an isolated incident; other regulatory approved therapeutics including blinatumomab, trastuzumab, alemtuzumab, muromonab & CAR-T cells also have documented similar (but lower) rates of infusion-related CRS.
“Where there are signs of cellular activation, in vitro studies should be undertaken[GJ1] to determine if the test therapeutic has the potential to induce toxicities in the clinic, regardless of negative findings from preclinical toxicology studies.” – FDA
What are Cytokine Release Assays (CRAs) and how do they work?
Cytokine Release Assays (CRAs) are an in vitro assessment utilizing immune cells collected from healthy subjects. The assays are designed to detect if whole blood or isolated lymphocyte preparations, when dosed with test therapeutic, induce a cytokine storm. The responses obtained are directly measured against known positive and negative therapeutic compounds with similar or known mechanisms of action. Supernatant samples are recovered and stored after incubation with compound. For all assay formats, the routinely evaluated cytokines (IL-2, IL-6, IL-10, IFNγ and TNFα) are analysed using human cytokine array multiplex technology.
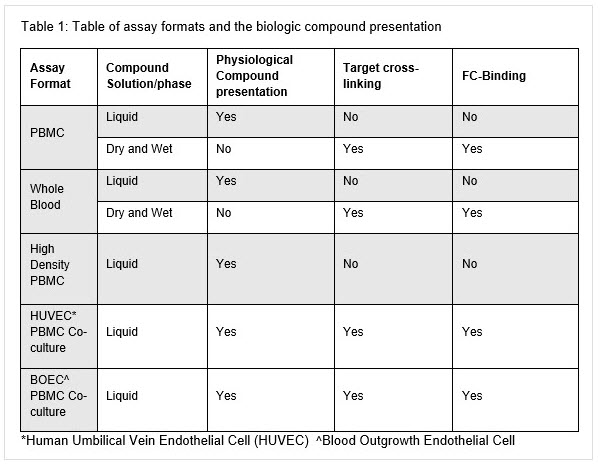
There are four main methodologies for assessing CRS in vitro:whole blood, peripheral blood mononuclear cells (PBMCs), high density PBMC, and blood outgrowth endothelial cell (BOEC) PBMC co-culture. Table 1 details the assay systems, target cross linking and solution/phase of therapeutic presentation. Further in-depth detail of the different assay systems will be described in a later blog post.
When are CRAs required?
In the example of TGN1412, the biologic was a monoclonal antibody; however, any small molecule or biologic which has the potential to interact with the immune system should be tested. Products targeting membrane receptors could pose a particular risk of promoting cytokine release.
Cancer immunotherapy is an increasingly popular area of drug development: many of the therapeutics also target the membrane-bound receptors which modulate immune function, so should be tested using a CRA.
What do regulatory guidelines recommend for CRAs?
Regulators have questioned the predictive value of preclinical testing strategies when testing novel biologic therapeutics. As such, it has become a regulatory expectation to conduct in vitro CRAs to de-risk biological therapeutic entities.
According to the FDA Guidance for Industry, Immunogenicity Assessment for Therapeutic Protein Products, “where there are signs of cellular activation, in vitro studies should be undertaken[GJ1} to determine if the test therapeutic has the potential to induce toxicities in the clinic, regardless of negative findings from preclinical toxicology studies.”
We offer PBMC, whole blood (liquid and wet coating, and dry immobilized) and high density PBMC assay options as a standard and other in vitro services to complement nonclinical and clinical development plans.
Read CRS Article Part II — Weathering Cytokine Storms: Different kinds of cytokine release assays.
About the Author
Chris Cooper, Scientific Specialist II, Immunotoxicology & Immunology
Labcorp Early Development Laboratories Ltd
The One Nucleus blog is written by individuals and is not necessarily a reflection of the views held by One Nucleus. Please email [email protected] for more information on 'guest blogging'.