By Alicia Gailliez, Business Development Manager, One Nucleus

Antibody drug conjugates (ADCs) show huge potential as a mode of targeted cancer treatment, as revealed recently in cancer trials. Take, for example, AstraZeneca and Daiichi Sankyo’s Enhertu, which significantly improved tumour response rate and overall survival in HER2-positive metastatic gastric cancer in the Phase II DESTINY-Gastric01 trial. Enhertu cut the risk of death by 41% compared with chemotherapy. An analysis published in the New England Journal of Medicine showed that patients treated with T-DM1 had a 50% lower risk of recurrence of invasive breast cancer or death than with trastuzumab alone.
ADCs are made by attaching a cytotoxic drug to a monoclonal antibody (MAb) using a synthetic linker. The goal is to combine the high toxicity of small drugs with the high specificity of antibodies to target cancer cells. An article entitled Antibody drug conjugate: the “biological missile” for targeted cancer therapy from the journal Signal Transduction and Targeted Therapy illustrates the key components of an ADC drug as per the diagram below.
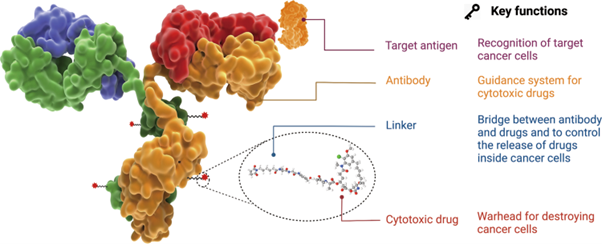
Image Credit: Fu, Z., Li, S., Han, S. et al. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Sig Transduct Target Ther 7, 93 (2022). https://doi.org/10.1038/s41392-022-00947-7 (online)
In their 2024 Drugs to Watch report, Clarivate identified ADCs as a modality likely to attain blockbuster status by 2029 or provide game-changing results to patients. The report highlighted a specific ADC Datopotamab deruxtecan (Dato-DXd), developed by AstraZeneca and Daiichi Sankyo for both HR-positive/HER2-negative and triple-negative breast cancer and non-small cell lung cancer (NSCLC). It is clear that Astra Zeneca is placing strong bets on in-house production of ADCs with the recent announcement that it intends to build a $1.5 billion manufacturing facility in Singapore for ADCs with targeted operational readiness from 2029. Other Big Pharma expanding their ADC pipelines include Eli Lilly and Bristol Myers Squibb.

 A recent Roots Analysis report estimates the global ADC market to be worth $7.72 billion in 2023 and is anticipated to grow at a CAGR of around 10% between 2023 and 2035, citing 14 approved ADCs for therapeutic use by the US FDA.
A recent Roots Analysis report estimates the global ADC market to be worth $7.72 billion in 2023 and is anticipated to grow at a CAGR of around 10% between 2023 and 2035, citing 14 approved ADCs for therapeutic use by the US FDA.
There are still plenty of challenges that exist around innovations in the ADC space. A BMC journal article entitled Antibody drug conjugates: hitting the mark in pancreatic cancer? published late last year indicates work to be done in fine-tuning ADCs, such as improved tumour targeting, linker chemistries, and payload properties. There are other generic challenges to consider as well as those associated with the broader spectrum of novel modalities entering the market. These could include off-target effects and safety concerns, for example. Trends show that pipelines are becoming more dominated by modalities outside of small molecules. A 2023 Nature article cites that new modalities such as antibody–drug conjugates, bispecific proteins, and cell and gene therapies accounted for approximately a third of new drug approvals in the US in 2022, helping push biologics approvals ahead of small molecules for the first time.
For this reason, our session at ON Helix ‘New Horizons for Therapeutic Modalities’ will talk about overcoming such associated challenges to help accelerate the future development of novel therapeutic modalities, including those that hold the power for transformative treatments as described above. This article explores some of the themes that will be discussed during ON Helix to give attendees a preview of what to expect.
A PharmaVentures White Paper on Value Creation in the CDMO Market highlights that the global biologics market is approaching a third of the entire pharma services market, to which CDMOs are reacting with more diverse manufacturing services to offer solutions to customers. Collaboration plays a significant role in risk sharing when exploring the development of new modalities whether that be a small molecule or biologic drug. It will be interesting to hear keynote speaker Kia Pedersen of Pelago Bioscience discuss reducing the risk of drug candidates failing in later stages through applying target engagement studies using their patented Cellular Thermal Shift Assay (CETSA).
Showcasing some of the exciting technologies to watch for emerging modalities will be Resolution Therapeutics and Harness Therapeutics. It will be interesting to hear Amir Hefni of Resolution Therapeutics talk about the science behind the development of their cell treatments to repair organ damage – including end-stage chronic liver disease based on a proprietary platform of macrophage biology, cell engineering, and manufacturing processes. As with ADCs, regenerative medicine holds promising investment potential for transforming healthcare, and one doesn’t have to look far to find local exemplars in this space with the likes of key players such as Altos Labs and Shift Bioscience.
However, a diverse range of new modalities can also present new challenges in clinical translation. The economic cost of engineered therapies, such as macrophage cell therapy, is a significant consideration. ON Helix promises to shed further light on this and other areas to be considered when developing these products to have a significant healthcare impact across a range of diseases, such as Cancer Immunotherapy.
 Also featured in our expert speaker line-up is Madeleine Wakeling at Harness Therapeutics who are using mRNA-targeted ASOs (and other oligonuclecotide-based modalities) to ‘harness’ the post-transcriptional regulation of protein synthesis and thereby elevate expression of a target protein in a controlled manner. Madeleine will discuss the numerous associated challenges, such as creating precise assays to detect subtle changes in protein expression; the necessity for human disease-relevant cell models to demonstrate efficacy; and ensuring safe delivery to patients, as well as the broader challenges such as chemistry, immunogenicity ,and safety studies.
Also featured in our expert speaker line-up is Madeleine Wakeling at Harness Therapeutics who are using mRNA-targeted ASOs (and other oligonuclecotide-based modalities) to ‘harness’ the post-transcriptional regulation of protein synthesis and thereby elevate expression of a target protein in a controlled manner. Madeleine will discuss the numerous associated challenges, such as creating precise assays to detect subtle changes in protein expression; the necessity for human disease-relevant cell models to demonstrate efficacy; and ensuring safe delivery to patients, as well as the broader challenges such as chemistry, immunogenicity ,and safety studies.
Our speakers, along with Benjamin Taylor, AstraZeneca and Davide De Lucrezia, Officinae Bio will participate in a panel discussion moderated by Hugh Nuthall, Eli Lilly. Attendees can look forward to further insights and understanding on the most promising modalities gaining traction and how to address the challenges of their clinical translation, the evolution of tools to ensure evaluation of efficacy, safety, and other critical aspects. The panel will also discuss how forming relationships with the right stakeholders early can support solving those technical challenges and embracing a new regulatory landscape.
Check out the full conference programme and make sure that you don’t miss out on the opportunity to connect with thought leaders discussing the latest developments in New Horizons for Bio Innovation by registering for ON Helix today!
