Bringing a novel therapeutic from concept to candidate is a complex journey, but what if you could streamline that process without compromising quality? In this case study, discover how BioDuro’s integrated drug discovery model helped a biotech partner progress from hit identification to a preclinical candidate in just 16 months.
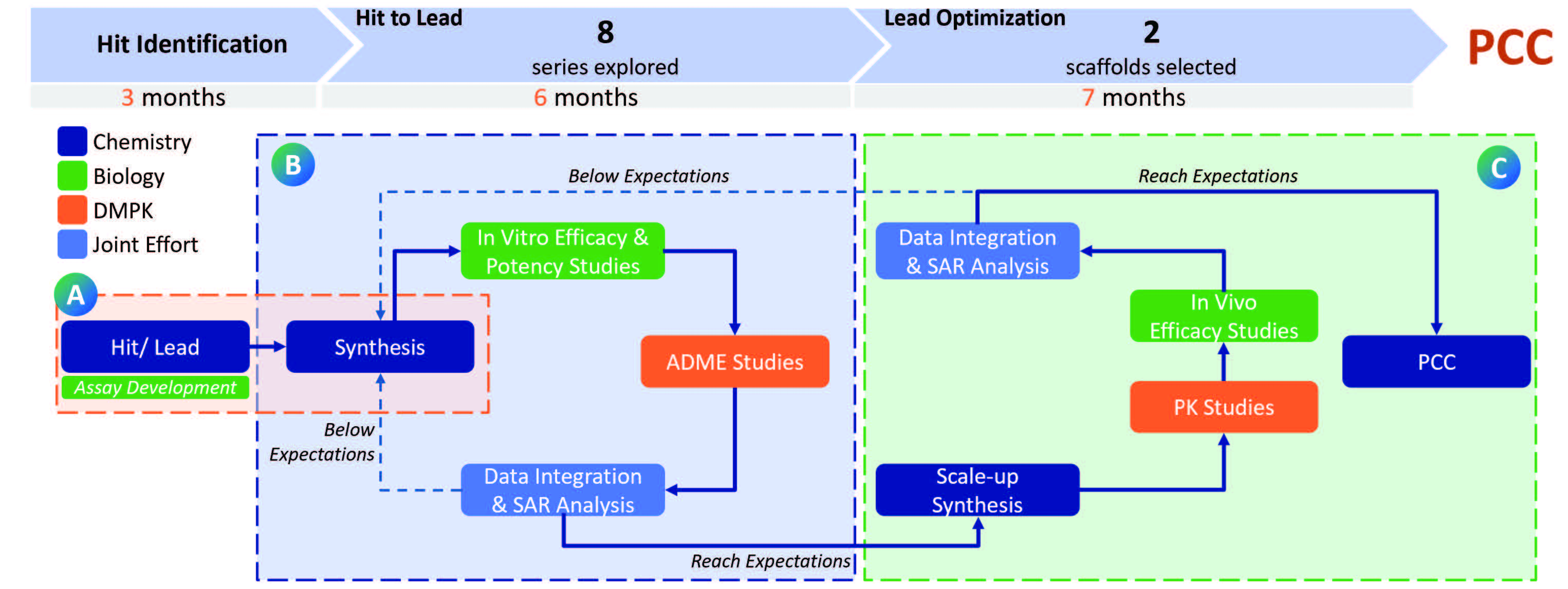
BioDuro successfully helped a biotech customer advance a kinase inhibitor for inflammatory disease from hit identification to a preclinical candidate (PCC) in just 16 months. The project was executed in three distinct phases — 3 months for Hit Identification (step A), 6 months for Hit to Lead (step B), and 7 months for Lead Optimization (step C) — showcasing an efficient and streamlined integrated drug discovery approach.
For this project, the BioDuro team synthesized and tested over 400 compounds, with all services completed at a single site. This setup increased efficiency in terms of material transfer, knowledge sharing and team collaboration. To ensure the most efficient process, all team leaders actively participated in regular meetings with the customer, facilitated by a dedicated project manager, enabling timely and effective communication.
A) Hit Identification
Medicinal Chemistry and Biology: Medchem design and synthesis of several fluorophores resulted in finding the right fluorophore for development of pseudokinase binding assay, which was used extensively in the project. In addition, 6 other kinase binding assays were developed for counter screening for selectivity.
B) Hit to Lead: 8 explored series of compounds
Medicinal Chemistry and CADD: Medicinal chemists and CADD team work together to convert hit compounds through core hopping and in silico calculations to pick best compounds for synthesis, in an iterative fashion, to make as many leads with good properties as possible – resulting in 8 series of leads.
Biology: Once synthesized, compounds are handed to the Biology team at the same site for in vitro profiling for SAR analysis.
DMPK: Conduct ADME studies to evaluate how compounds are absorbed, distributed, metabolized and excreted.
The compiled biology data is then shared with the client for evaluation and further refinement. As needed, the Chemistry team modifies the molecular structures based on client feedback to optimize potency, selectivity and other drug-like properties to meet goals set for each milestone.
These improved compounds re-enter the testing cycle, ensuring steady progress. Once meeting the customer’s requirement, the project moves into Lead Optimization stage (LO) for further development.
C) Lead Optimization: 2 selected scaffolds
Chemistry: Continue to synthesize and test compounds in vitro biology and in vitro DMPK studies. Compounds meeting potency and in vitro DMPK are scaled-up to produce larger quantities of lead candidates for PK studies.
Biology & DMPK: In vivo studies and PK studies are conducted to assess their in vivo efficacy potential, respectively.
Similar to step B, the process continues until the most promising compounds are identified as Preclinical Candidates (PCCs) to enter the IND-enabling stage.
Through precise workflows, data-driven decision-making, and full-circle integration across discovery stages, we significantly reduced timelines without compromising scientific rigor.
To read the full case study, click here
*Guest blog by BioDuro. If you would like to submit a guest blog please email [email protected]